The role of carbohydrates in alternate bearing in pecans
by Dr Elmi Lötze (Head of ITEST™CARBOHYDRATES and ITEST™LEAF) and Deon Claassen (Senior Horticulturist)
Alternate bearing
Carbohydrates play an important role in the alternate bearing cycle of many fruit types, including pecan trees. Alternate bearing refers to the phenomenon where trees produce a large crop in one year (the so-called on-year) and a low harvest the following year (off-year).
The appearance of alternate bearing varies and may involve a branch, tree, or orchard (Monselise and Goldschmidt, 1982). It also does not necessarily follow a two-year cycle, as two to three on or off-years may occur in succession (Wood et al., 2003).
The most important economic challenge in pecan production globally is alternate bearing (Wood et al., 2003). Any manipulation to reduce or entirely prevent its occurrence, will therefore significantly contribute to the successful (and economical) cultivation of pecans.
A method used to quantify alternate bearing is calculating an alternating index, which determines the intensity of alternate bearing. Intensity ranges from 0, where yields remain more or less constant between years, to 1, where complete alternation occurs in a year with no yield (Conner and Worley, 2000).
Alternatively, an accurate crop prediction model will not only predict the possibility of alternation, but also quantify the increase or decrease in production and thus support planning going forward.
Factors that determine alternation
Alternate bearing is regulated by nutrition, hormones and the carbohydrate status of the tree. The occurrence of alternate bearing in pecan trees is mainly due to three energy-related factors: the lengthy period it takes for nuts to reach maturity, fruit growth/enlargement and the chemical composition of the kernel (Sparks, 1974).
Carbohydrates are associated with most phenological stages during nut development – from flower induction to harvest. According to the Carbohydrate Theory, flower and fruit formation are directly determined by the carbohydrate reserves that build up during the dormant period (Barnet and Mielke, 1981).
In contrast, in terms of the Phytohormone Theory, these processes are controlled by hormones via the fruit, leaves, or both. Furthermore, a close relationship exists between the transport of hormones and sugar and the hormonal signal for biochemical reactions. Flower induction, as an example, must be supported by energy derived from carbohydrates for the final outcome, for example, a reproductive bud.
Flower development and fruit growth (nut) are proportional to the carbohydrate reserves that have built up during the dormant season. Cultivars that reach maturity early are able to accumulate carbohydrates over a longer period of time and are therefore less subject to alternate bearing than late maturing cultivars.
The kernel accumulates approximately two-thirds of its total dry weight during the 80 days before leaf drop and contains a high percentage of lipids (~70%). This will require the mobilisation of a significant amount of energy during the short period before leaf drop in order to meet this requirement (Monselise and Goldschmidt, 1982). Carbohydrate reserves therefore need to be replenished in time i.e., before and after this period of nut fill and also dormancy, that follows thereafter.

Figure 1: Water stage of pecan nut in March.
Carbohydrate management and alternate bearing
During an on-off year, a large portion of the carbohydrates produced by photosynthesis are consumed for nut development and growth. The result is that carbohydrate reserves in the tree, especially starch, are significantly reduced which can lead to a decrease in vegetative growth and inhibit flower bud formation in the following year. In the off-year the crop load is reduced, the tree has an excess of carbohydrates. This in turn results in the accumulation of starch reserves, promoting vegetative growth and the formation of flower buds for the next year’s harvest.
Photosynthesis
The size of the active energy and reserve energy pool is related to the photosynthetic capacity of the leaves during the season (Conner and Worley, 2000). Thus, the first priority of the producer is to ensure a healthy leaf surface with adequate light interception and distribution in the orchard. Since water and mineral elements are also required for photosynthesis, irrigation scheduling and a focused fertilisation strategy are critical. All these practices impact the physiological status of the tree.
Carbohydrate analyses
An excellent way to quantify this status is to take carbohydrate samples for analysis at critical action times and then interpret the results in terms of the historical information to determine future management actions. The carbohydrate status of roots and shoots at the end of the dormant season may therefore be indicative of i) the post-harvest strategy to replenish carbohydrate reserves, ii) the utilisation of reserve carbohydrates during the winter and iii), what could be expected in the coming season in terms of available carbohydrates for bud break and the initial growth in spring.
Low starch levels in the tissues during this stage is an early-on indication that photosynthesis should be stimulated early and, in case a large crop sets, that follow-up support is going to be essential.
To increase carbohydrate levels for the following year, various manipulations can be applied at farm level to ensure optimal photosynthesis and the transport and distribution of sugars. These include pest management, increased sunlight interception and distribution through pruning practices and plant density, good irrigation scheduling and plant nutrition.
Furthermore, manipulations such as girdling, plant growth regulator-induced growth stress and fruit set can also be used for carbohydrate and/or hormone management during set. The effectiveness of these manipulations varies between cultivators, time of execution and intensity.
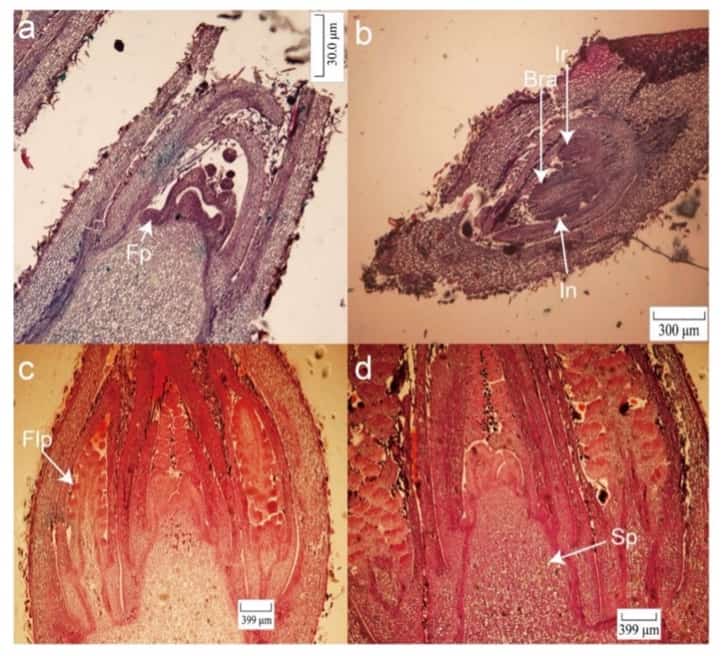
Figure 2: Morphological characteristics of early male flower bud differentiation of ‘Mahan’, China (Xie et al. 2023).
(a) Early stage of differentiation with flowering primordia (Fp) late October (RSA).
(b) Male flower primordia with casing (In), bract (Bra) and inflorescence rachis (Ir) from mid-October.
(c) Flower primordia (Fp) appears along with the elongation of the male inflorescence and increase in bracts in early January.
(d) The stamen primordium (Sp) becomes visible after calyx primordium differentiation at the end of October.
Pilot trial
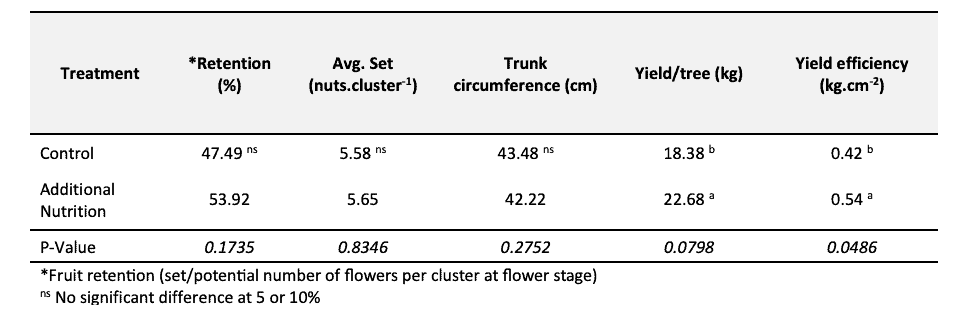
In a promising, local pilot trial (Snyman, 2021), an additional nutrient foliar spray during March (year 1 & 2) on a block with no nutrient deficiencies (leaf analysis), followed by a soil fertilisation/seaweed/fulvic acid combination in September (year 2), resulted in a significant increase in yield (P<0.10) and yield efficiency (P<0.05) on young (5 – 6 years), bearing ‘Wichita’ the following season (year 2) (Table 1).
Table 1. Tree performance of a pilot trial with additional leaf and soil applications in March (leaf) and September (both), with no nutrient deficiencies on young, bearing ‘Wichita’ trees at Prieska 2018/9, adapted from Snyman (2021).
The application in March (water stage (Figure 1) late differentiation stage of male flowers of subsequent year (Figure 2)) may have played a role in photosynthesis, which could support the build-up of reserves as well as nutritional elements thereafter for the 2nd year harvest. The supplementation in September (bud swelling (Figure 3) differentiation of female flowers from next year (Figure 4)) plays a role in managing energy for the harvest in year 2.
The morphological development of the male and female flowers for Mahan in China for around September (RSA) is illustrated in Figures 1 and 2 (Xie et al., 2023).

Figure 3: Bud swell of pecan tree.
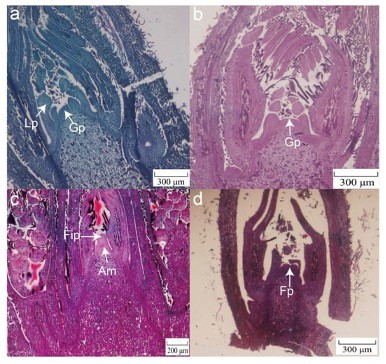
Figure 4: Morphological characteristics of female flower differentiation in ‘Mahan’, China (Xie et al. 2023).
(a) The growth point (Gp) of the bud becomes smaller and pointed and the leaf primordia (Lp) begins to differentiate – beginning of November (RSA)
(b) Growth points of flower buds flatten and buds reach the critical period of differentiation which extends to the end of December.
(c) From late December to mid-January, bud growth points extend upward and form a prominent sphere. The apical meristem (Am) and female inflorescence primordia (Fip) become visible.
(d) After more morphological changes, the protrusions in female primordia (Fp) differentiate.
The effect of the treatments on the carbohydrate status of the tree and therefore yield effectiveness, was not quantified in the thesis. Agri Technovation plans to start the upcoming season with a similar approach including quantification with the ITEST™CARBOHYDRATES service on commercial orchards.
References:
- Barnett, J. and Mielke, E.A. 1981. Alternate bearing: A re-evaluation. Pecan South 8: 20-30.
- Conner, P.J. and Worley, R.E. 2000. Alternate bearing intensity of pecan cultivar HortScience 35: 1067-1069.
- Monselise, S.P. and Goldschmidt, E.E. 1982. Alternate bearing in fruit trees. Horticultural Reviews 4: 128-173.
- Snyman, T.J. 2021. Investigating the reproductive potential and yield of Pecans [Caryaillinoinensis (Wangenh. K. Koch)] under South African condition MSc Agric thesis, Stellenbosch University.
- Sparks, D. 1974. The alternate fruit bearing problem in pecans. Annual Report, Northern Nut Growers Association 47: 145-158.
- Wood et al. 2003. Relationship of alternate bearing intensity in pecan to fruit and canopy characteristics. HortScience 38: 361-366.

